A PHP Error was encountered
Severity: Warning
Message: ini_set(): Session ini settings cannot be changed when a session is active
Filename: Session/Session.php
Line Number: 284
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: session_set_cookie_params(): Session cookie parameters cannot be changed when a session is active
Filename: Session/Session.php
Line Number: 296
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: ini_set(): Session ini settings cannot be changed when a session is active
Filename: Session/Session.php
Line Number: 306
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: ini_set(): Session ini settings cannot be changed when a session is active
Filename: Session/Session.php
Line Number: 316
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: ini_set(): Session ini settings cannot be changed after headers have already been sent
Filename: Session/Session.php
Line Number: 317
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: ini_set(): Session ini settings cannot be changed after headers have already been sent
Filename: Session/Session.php
Line Number: 318
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: ini_set(): Session ini settings cannot be changed after headers have already been sent
Filename: Session/Session.php
Line Number: 319
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: ini_set(): Session ini settings cannot be changed after headers have already been sent
Filename: Session/Session.php
Line Number: 377
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: ini_set(): Session ini settings cannot be changed when a session is active
Filename: drivers/Session_files_driver.php
Line Number: 108
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Warning
Message: session_set_save_handler(): Session save handler cannot be changed when a session is active
Filename: Session/Session.php
Line Number: 110
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
A PHP Error was encountered
Severity: Notice
Message: session_start(): Ignoring session_start() because a session is already active
Filename: Session/Session.php
Line Number: 143
Backtrace:
File: /home2/amanaayg/public_html/lithiumionbattery.org/application/controllers/Activities.php
File: /home2/amanaayg/public_html/lithiumionbattery.org/index.php
Chemistry – Lithium Ion Battery
Chemistry – Lithium Ion Battery
VIDEO
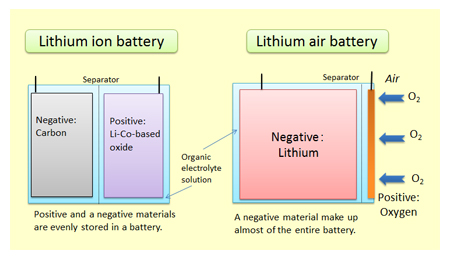
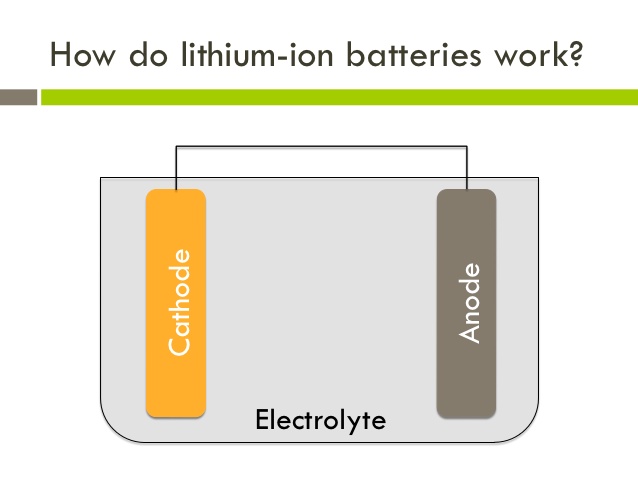
The three participants in the electrochemical reactions in a lithium-ion battery are the anode, the cathode, and the electrolyte. Both the anode, which is a lithium-containing compound, and the cathode, which is a carbon-containing compound, are materials into which lithium ions can migrate. The electrolyte is a lithium salt in an organic solvent. When a lithium-based cell is discharging, the positive lithium ion is extracted from the cathode and inserted into the anode, releasing stored energy in the process. When the cell is charging, the reverse occurs.




How Lithium-ion batteries are made